Modeling Patient Engagement Throughout TPL
Overview
Provide framework and pathway for industry to incorporate patient input and patient centricity throughout device development and each stage of the Total Product Lifecycle (TPL).
Objective
Increase systematic Patient Engagement tools and processes throughout the Total Product Lifecycle (TPL)
What is the TPL?
| As noted in the diagram, the pathway to successful device development is an iterative process, with ideas generated, tested, improved, re-tested, optimized, and finalized.
Throughout the total product lifecycle (TPL), device developers and regulators take steps to ensure that a new device can be safe and effective when used by the patient population for which it has been designed. |
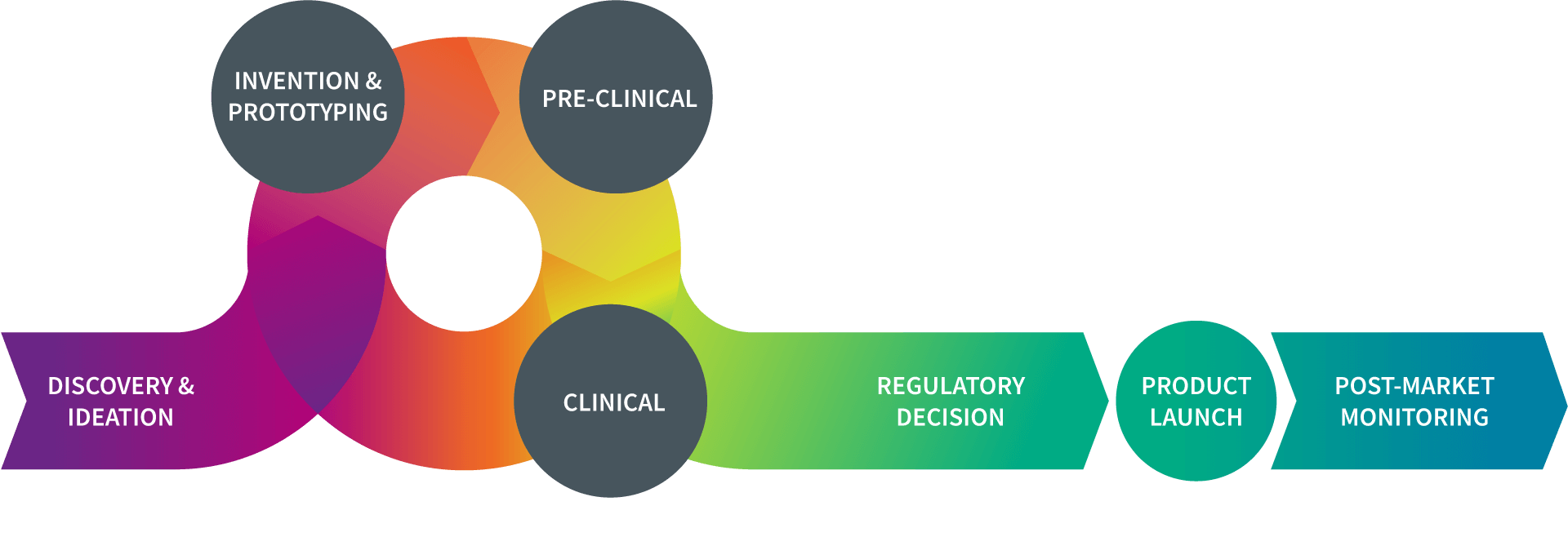
When confronted with an unmet medical need, researchers in the device field will come up with an idea for a new product. Once they have a “proof of concept” plan, they will evaluate whether the idea is workable. Those concepts that exhibit promise move forward toward the next stages of development.
In the invention phase, researchers in the device field build a prototype or early version of the device to lay the foundation for further development. This prototype provides a device that can be tested in a lab. At this stage, the device prototype is not for human use.
The device prototype is evaluated in a controlled laboratory setting to provide researchers with important information about how the product may perform once it used in people. By refining the device during this phase, researchers work to reduce as much risk of harm as possible once the product moves into human use.
The term “clinical research” refers to studies, or trials, in which the device is tested in humans. Once a sponsor has sufficient pre-clinical data, the next step is to plan for clinical studies to establish the safety and effectiveness of the product.
Once a device has received marketing authorization from the FDA, there are additional steps the sponsor must take in preparing to make the device available for public use. This includes finalizing the “device master record” (DMR), as well as setting up coverage submissions, payor enrollments, a network for distributing the product to providers and the public, developing marketing materials, and registering with the FDA.
Even after granting marketing authorization for a medical device, the sponsor continues to monitor how it performs once it is commercially available. Although pre-market clinical trials provide important information on a device’s safety and effectiveness, it is possible new safety concerns will emerge. Post-marketing surveillance is critically important to ensuring patient safety.
Active Working Groups
Modeling Patient Engagement in Early Phases of Device Development
The Early Phase group is focused on mapping out various types of Patient Engagement and Patient Preference Information (PPI) at various stages of the device TPLC. Developing a framework detailing how, when, where, and what type can be incorporated in product development.
- Subgroup 1: Clinical Innovation
- Subgroup 2: Device Design
Patient Engagement within Post Market Surveillance Practices
The Post Market Working Group is devoted to developing recommendations for three aspects of post-market patient engagement:
- Safety Communication: Communication regarding safety issues
- RWE: Use of Real-World Evidence (RWE) to meet regulatory requirements
- Benefit/Risk: Use of patient preference information to frame benefit-risk assessment
Framework for Direct Patient Input (DPI) in Real World Data and Evidence Generation
The Direct Patient Input in Real World Data Framework from MDIC provides a structured approach to embedding patient perspectives into real-world evidence generation throughout the medical device lifecycle.
This resource supports the collection and standardization of patient input through tools such as wearables, digital platforms, and patient-reported outcomes. It includes best practices for capturing diverse voices, strategies for improving data quality and usability, and insights into how artificial intelligence can transform raw data into meaningful evidence. The framework is designed to enhance clinical trial design, post-market surveillance, and regulatory decision-making by making patient input a central component of evidence development.

Elevating Your RWE Strategy With Direct Patient Input
Science of Patient Input (SPI) Survey on Digital Health Technologies and Patient Input
Using the MDIC Patient-Centered Benefit-Risk Framework to Support an Expanded Indication—a Case Study
Science of Patient Input (SPI) Program
Check out the SPI Home to learn more about SPI projects, initiatives, news, upcoming events.