Overview of the Program:
Introduction Statement
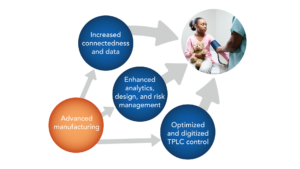
Advanced Manufacturing in medical devices is an approach that uses innovative techniques that applies the use of electronic information, computer technology, machinery, materials, and tech-enabled management to improve the quality and process of production. Each organization has unique needs and starting points in the journey toward an advanced manufacturing process.
Mission Vision and Impact
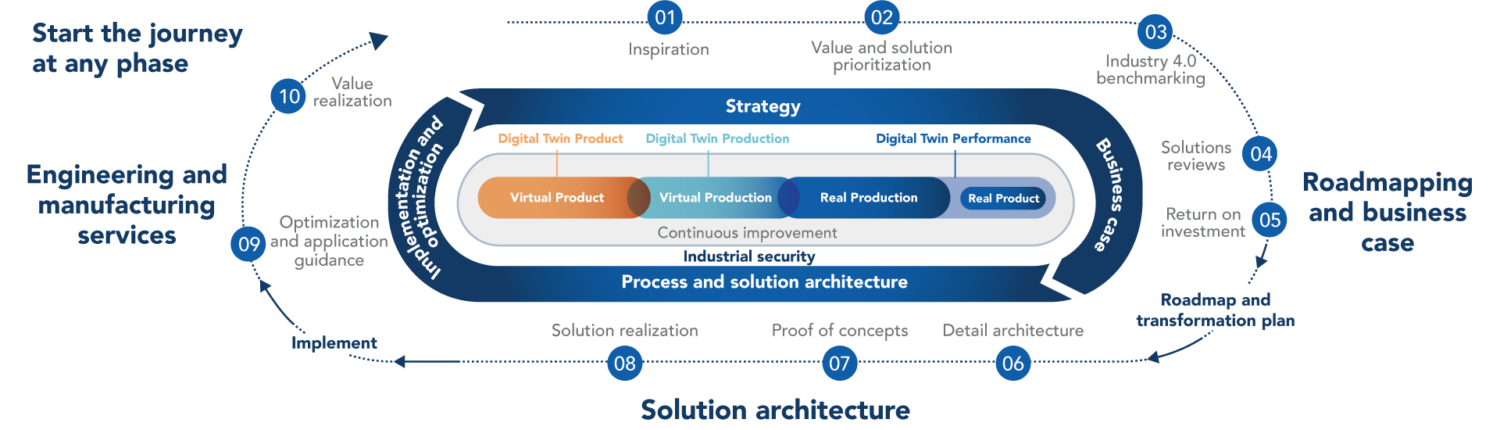
The purpose of Advanced Manufacturing Clearing House is to encourage Medical Device Industry to adopt advanced technology across the total product life cycle. Objectives include improving design, supply, production, distribution, tracking, device reliability, overall quality, and safety across the product life cycle. Examples of advanced technologies include but not limited to additive and generative part design, digital twins, and digital threads (e.g., modeling and simulation for virtual design verification, validation and design transfer), and data-driven closed-loop quality.