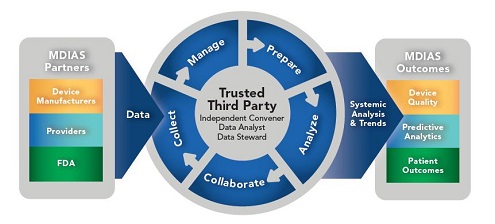
A wealth of data are collected during the medical device development process and in real-world environments. But these data vary in quality and format and are currently siloed within organizations for privacy and proprietary reasons. Developing an independent third party to collect and analyze these data can help identify trends in device quality and safety and provide powerful insights for improving outcomes while safeguarding data.
CfQcc Medical Device Information Analysis & Sharing (MDIAS)
The MDIAS Initiative focuses on building a data-sharing collaboration to analyze and share medical device data from various public and non-public sources to improve healthcare outcomes. Data will be safeguarded by an independent trusted third party (TTP) to foster broad participation and engagement.